Shipping Diagnostic Specimens
All diagnostic specimens, including blood and blood products, tissue and tissue fluids, excreta, and secreta, must be packaged using an IATA and DOT compliant triple packaging system that prevents leakage or damage.
- Primary containers must be watertight for liquid specimens. Individual containers must be wrapped or separated in a way that prevent potential damage. Absorbent material, preferably cellulose wadding, should be located between the primary and secondary containers. Urine containers are discouraged for the transport of liquid specimens; please remove liquid specimens to a labeled conical tube for transport. Screw top, snap-on and push-on lids should be sealed with paraffin laboratory tape such as ParafilmTM.
- Secondary container must be watertight but is not required to be rigid. A sealed plastic bag is acceptable.
- Outer packaging must be rigid and constructed of sturdy fiberboard, plastic or other solid material. Diagnostic specimens should be marked “Exempt Human Specimen” or “Exempt Animal Specimen” in compliance with IATA specifications.
Dried blood spots and fecal occult blood screens are not subject to regulations as infectious substances and require no special markings for shipment. Use only designated absorbent paper specimen collection systems for the collection, storage and transport of dried blood spots.
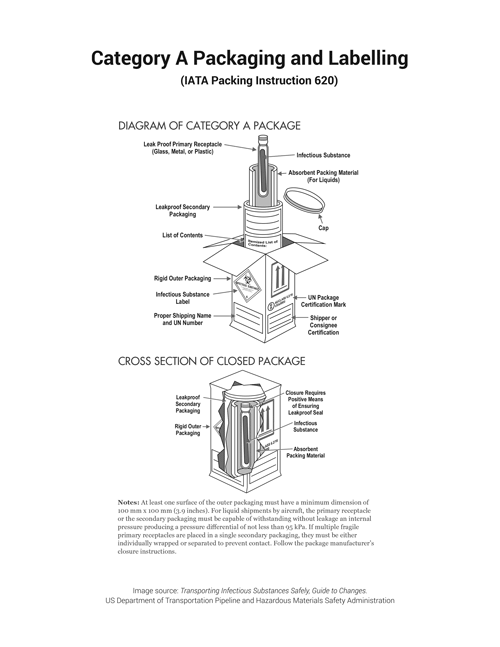
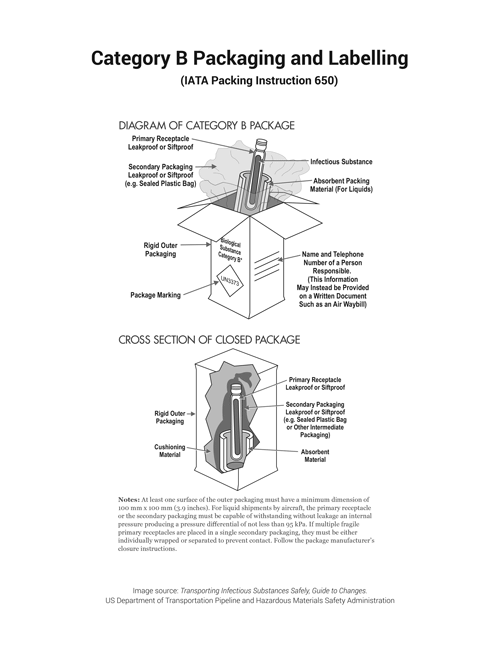
Shipping Category A and B Specimens
Follow the most current IATA or DOT classification requirements for classifying specimens as Category A, B or Exempt. Reference the following Checklist for guidance in Category A and B packaging. These checklists are not intended to be a sole resource when preparing shipments and persons performing shipping and packaging of infectious substances must have received training appropriate to the level of the task. If elements in these checklists are missing for any package containing a Category A or B specimen, the package is improperly packaged according to SNPHL requirements and may be in violation of federal regulations. Packages that do not meet requirements may be rejected for testing.
Resources for Shipping Category A, B, and Exempt Specimens
Updated on: March 23, 2022